Coronavirus – What You Need to Know Part II
Cambiar Senior Analyst Charmaine Chan provides her second Q&A segment on COVID-19. She details the latest on possible vaccines, the next steps for the U.S. on the road to recovery, and the likeliness of a second wave of coronavirus.
Cambiar Senior Analyst Charmaine Chan provides her second Q&A segment on COVID-19. Ms. Chan details the latest on possible vaccines, the next steps for the U.S. on the road to recovery , school openings, and the likeliness of a second wave of coronavirus.
Transcript:
Where do we stand with a possible vaccine?
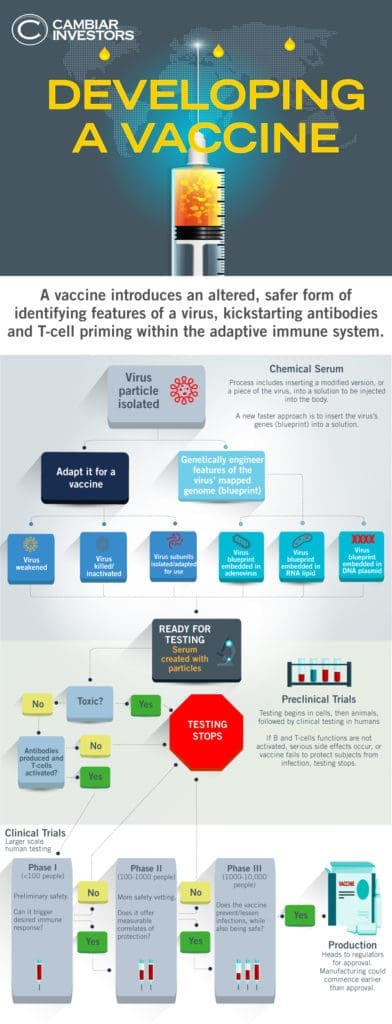 In the past weeks, more in-depth characterization of the virus, our immune responses post infection, as well as preliminary Phase I trials suggest we are more likely to successfully develop a vaccine than 2 months ago. These vaccines are likely to lessen the severity of infections, as opposed to preventing COVID altogether. The market is expressing this optimism.
In the past weeks, more in-depth characterization of the virus, our immune responses post infection, as well as preliminary Phase I trials suggest we are more likely to successfully develop a vaccine than 2 months ago. These vaccines are likely to lessen the severity of infections, as opposed to preventing COVID altogether. The market is expressing this optimism.
Whether this optimism is warranted depends on how efficacious and how safe these vaccines would be, as clinical data emerges in the next 6-9 months. The market will answer the efficacy question with Phase II data in the Fall, and the safety question with Phase III data late 2020/early 2021. Vaccines first and foremost need to be safe, as they are administered to healthy people, but note the safety question will be answered in the last stages of clinical trials when thousands of people have been tested.
When you hear that there are over 100 vaccines in development and 10 in clinical trials, you get the impression that one or more are bound to work. What we would note is that while there is a diversity in vaccine approaches (e.g. harmless viruses carrying genetic material of nCoV2, encapsulated DNA, RNA or viral antigens w/ adjuvants), the field is highly focused on one target – namely the spike protein, with good rationale. It is how tightly the spike protein binds to the ACE2 receptor on cells that makes the virus so infectious, so many are betting that as mutations occur as the virus multiplies and spreads, that the surviving strains will continue to feature the same spike protein, increasing the chances that our vaccines will work for the long haul. Developing vaccines using this one identifying piece of the virus is helping to speed up the progress, but we run the risk that the resultant vaccine could only be good enough, and may or may not adequately protect the most vulnerable populations.
Secondly, when we hear of availability of vaccines in the Fall, this refers to vaccines being manufactured at risk as we speak, before we have Phase II or Phase III data proving they are safe/efficacious. Vaccine availability does not equate to mass deployment and that consumers will get vaccinated. The likely path is if PII data is successful in the Fall, regulatory agencies around the world would consider approving vaccines for populations at highest risk of exposure (e.g. healthcare workers, essential workers, public-facing civil servants). It is unlikely, in our view, given that safety is paramount, that vaccines will be approved/made available for the general public until after we have successful PIII data in late 2020/early 2021.
Is social-distancing working? What are the other public health tools we have?
Yes, social distancing has worked. The shelter-in-place was an extreme form of social distancing that has reduced transmission rates by reducing the number of people each of us encounter. Epidemiologists estimate it has reduced Ro (the basic reproduction number, or new secondary cases from each existing case) from 2-3 to <1. Keeping Ro <1 is key to having new cases flat or declining, or the pandemic in control.
Reopening of states is transitioning to a more sustainable form of social distancing. The more relaxed social distancing becomes, we believe the more likely Ro will be >1. If Ro stays above 1 for a sustained period of weeks, it is highly likely we will see increasing new cases, or potentially exponentially growing cases, as is the case with Mexico and Brazil currently, where we see hospital runs.
While we believe social-distancing is the most effective tool, we must complement it with broad-testing and contact tracing to bring effective Ro as close to zero as possible. Countries that have been the most effective in controlling the pandemic successfully employed all 3 measures.
The U.S. has made progress in ramping daily testing capacity to 300-400K/daily, with daily test positivity rate coming down from 20%+ to 5%, the recommended levels in the CDC guidelines. However, it is unclear whether all the added test capacity is RT-PCR or serology. The former is to test for presence of genetic material of the virus, given to people suspected of COVID exposure. Identifying quickly who is infected helps stop transmissions if we could isolate/treat them as early as possible. We could broaden testing even further by pooling samples and doing subsequent rounds of testing. Serology is to test for presence of antibodies to confirm someone has had COVID infection. Note that since the prevalence of COVID infection is still relatively low in the U.S., even highly sensitive and specific serology tests could produce a large proportion of false positive results (e.g. 99% sensitivity and specificity with <5% prevalence will have positive predictive value of 90%, with 10% of positives as false positives), making serology tests less useful as a public health tool at this stage.
Contact tracing goes hand in hand with increased testing to lower transmissions. We believe the U.S. is behind in hiring, training and expanding contact tracing workforce (30 – 60 tracers/100K population, or national team of 100-200k), ramping only to ~66K in the coming weeks as states are re-opening. Tracers are there to interview the infected, identify businesses where they might have been exposed (for sanitation), people they may have exposed, and convince them to get tested and to self-isolate for at least 2 weeks. We feel speed is of the essence as the newly infected could be infectious up to 5 days (incubation time 5-14 days) before symptoms appear. Tracers also act as counsellors to ensure the newly exposed have the physical, financial and emotional resources to forgo work/socialization to self-isolate. High density of both RT-PCR testing and tracers increase the probability we could identify clusters, test and isolate the infected within days of exposure to limit further spreads. If social distancing reduced the transmission factor (R0) to close to 1, we believe widespread testing and contact tracing could bring Ro closer to 0.5, allowing governors more leeway to relax social distancing measures for potentially faster economic recovery.
Will we likely see a second wave?
Cambiar believes that there are likely going to be an increase in new cases before PII data readout in Fall 2020. Note that higher temperatures, humidity and people spending less time in closed spaces indoors is likely helping to lower transmissions currently, but we feel the relaxing of social distancing behaviors is likely occurring too quickly. We are testing more broadly, but likely still not optimally, and we are not adopting comprehensive contact tracing. Recall that the rate of change in new cases correlate to 3 factors: the transmission rate (which is dynamic dependent on behaviors and public health tools), the pace at which infected individuals stop becoming infectious (which is static, 14-30 days) and the number of existing infectious individuals. The lockdown has reduced the number of infectious people currently. The analogy is we have lessened the seeds of infections, so it will take time to repopulate the infectious populations and hence the speed of new cases. The exact magnitude/timing of a 2nd wave is therefore variable, dependent on how well we retain social-distancing behaviors and how well broad testing, contact tracing and other public health measures are implemented. President Trump has primed our expectations that if there are ‘flares’ of higher new cases, select lockdowns will be ‘very targeted’. Asian and most European Union countries have done much better containment and risk of second waves is lower.
dIdt ≈ (β−γ)I
Rate of Change of (New) Infectious Cases ≈ (Transmission Rate – Recovery Rate) * Proportion of Population that is Infectious
Ro = β/γ
If kids are carriers, when they go back to school in the Fall, doesn’t that increase the probability of 2nd waves?
This is likely on the minds of every parent, for both the safety of their child and for their own sanity and productivity. So far, while children can certainly get COVID and manifest hyperinflammatory syndrome (PIMS-TS), they appear to catch it less often than adults based on studies of same-household transmissions. They also appear to have numerically lower viral loads vs adults, so on the margin they could be less infectious. Thus, it is possible they are less likely to catch COVID from one another at school, and potentially slightly less likely to transmit it to their parents/grandparents vs another adult. However, probability dictates that any large, interactive groups form the basis for infection clusters, so the risks clearly exist. Therefore test/trace/isolate is crucial to prevent large scale second waves.
Has Cambiar’s investment outlook changed based on the new information?
We believe some of the market recovery is well-grounded on several aspects: 1) the mortality rate is likely closer to 0.5 – 1% vs initial estimates of 2-3% with broader testing, more established clinical management protocols and the approval of Gilead’s Remdesivir as an antiviral, which shortens recovery time if given early in the treatment, and 2) better understanding of the virus and our adaptive immune responses (both B-cell and T-cell based) post infection has given us confidence that a vaccine is possible.
On the other hand, we believe the markets could be overlooking: 1) the morbidity, or long-term health effects to patients who recovered from COVID. While consumers want to resume some social/economic activities, the judicious ones are rightly displaying risk aversion to group activities in confined spaces, especially in countries where new cases have not consistently declined. 2) the vaccines that emerge in late 2020/2021 might not be as efficacious or as safe as we would like, limiting its usefulness and adoption, and hampering the much hoped full economic recovery and 3) while most investors expect a second wave, we believe there is a behavioral bias to be reactionary vs anticipatory. The markets are watching for any sustained increase in new cases, which by the time the trends are confirmed, the public health crisis will have already re-emerged.
Given this view, we strive to be balanced in our deployment of capital, in accordance with our philosophy of finding quality, durable businesses with low leverage that could withstand potentially another round of economic disruptions.
Source:
https://www.scientificamerican.com/article/genetic-engineering-could-make-a-covid-19-vaccine-in-months-rather-than-years1/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6962332/
https://www.voanews.com/covid-19-pandemic/trump-hopeful-coronavirus-vaccine-year
https://www.sciencemag.org/news/2020/05/why-do-some-covid-19-patients-infect-may-others-whereas-most-don-t-spread-virus-all
https://www.statnews.com/2020/05/15/trump-audacious-plan-vaccine-covid19/
https://www.pewresearch.org/fact-tank/2020/05/21/most-americans-expect-a-covid-19-vaccine-within-a-year-72-say-they-would-get-vaccinated/
https://investors.modernatx.com/static-files/e5fd7ac7-53c9-4804-b89d-4e51d5121960
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31208-3/fulltext
https://www.biorxiv.org/content/10.1101/2020.05.13.093195v1
https://www.nature.com/articles/s41467-020-16505-0https://coronavirus.jhu.edu/testing/individual-states/
https://www.fiercebiotech.com/medtech/fda-publishes-first-validation-results-12-covid-19-antibody-tests
https://www.news-medical.net/news/20200422/Antibodies-to-COVID-19-present-in-only-2-to-3-percent-of-population.aspx
https://www.cnn.com/interactive/2020/us/states-reopen-coronavirus-trnd/
https://www.medrxiv.org/content/10.1101/2020.05.19.20105999v1.full.pdf
https://www.technologyreview.com/2020/05/16/1001787/why-contact-tracing-may-be-a-mess-in-america/
https://www.medrxiv.org/content/10.1101/2020.05.05.20091280v1.full.pdf
https://www.politico.com/news/2020/05/17/privacy-coronavirus-tracing-261369
https://apnews.com/a391d11d256d475098a426ebccd89cbe
https://www.wired.com/story/i-enrolled-in-a-coronavirus-contact-tracing-academy/?utm_source=pocket-newtab
https://www.politico.com/news/2020/05/17/privacy-coronavirus-tracing-261369
https://jamanetwork.com/journals/jamapediatrics/fullarticle/2766037
https://zoonosen.charite.de/fileadmin/user_upload/microsites/m_cc05/virologie-ccm/dateien_upload/Weitere_Dateien/analysis-of-SARS-CoV-2-viral-load-by-patient-age.pdf
https://jamanetwork.com/journals/jama/fullarticle/2766524https://co.chalkbeat.org/2020/5/26/21271260/colorado-schools-fall-reopening-state-guidance
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6962332/
Certain information contained in this communication constitutes “forward-looking statements”, which are based on Cambiar’s beliefs, as well as certain assumptions concerning future events, using information currently available to Cambiar. Due to market risk and uncertainties, actual events, results or performance may differ materially from that reflected or contemplated in such forward-looking statements. The information provided is not intended to be, and should not be construed as, investment, legal or tax advice. Nothing contained herein should be construed as a recommendation or endorsement to buy or sell any security, investment or portfolio allocation.
Any characteristics included are for illustrative purposes and accordingly, no assumptions or comparisons should be made based upon these ratios. Statistics/charts may be based upon third-party sources that are deemed reliable; however, Cambiar does not guarantee its accuracy or completeness. As with any investments, there are risks to be considered. Past performance is no indication of future results. All material is provided for informational purposes only and there is no guarantee that any opinions expressed herein will be valid beyond the date of this communication.
